What we do
Climate change is the defining issue facing our generation and generations to come. Its underlying cause is clear - accumulated greenhouse gases, emitted from fossil fuel exploitation, absorb infrared radiation and cause global warming. However, a large but highly uncertain fraction of the warming has been masked by atmospheric aerosol particles, via their direct effect of reflecting incoming solar radiation and indirect effect of serving as seeds for cloud formation.
This uncertainty in the aerosol forcing is rooted in the unknown baseline aerosol state of the preindustrial atmosphere, and the poorly understood mechanisms driving the anthropogenic increase in aerosols and clouds to date. Our research aims to address this uncertainty and provide novel approaches to (a) constrain the oxidative chemistry of emerging pollutants for particle nucleation, (b) resolve microphysical processes in particle growth and survival, and (c) develop a particle dynamics model with experimental and meteorological inputs for real-world deployment.
Oxidative chemistry and volatility
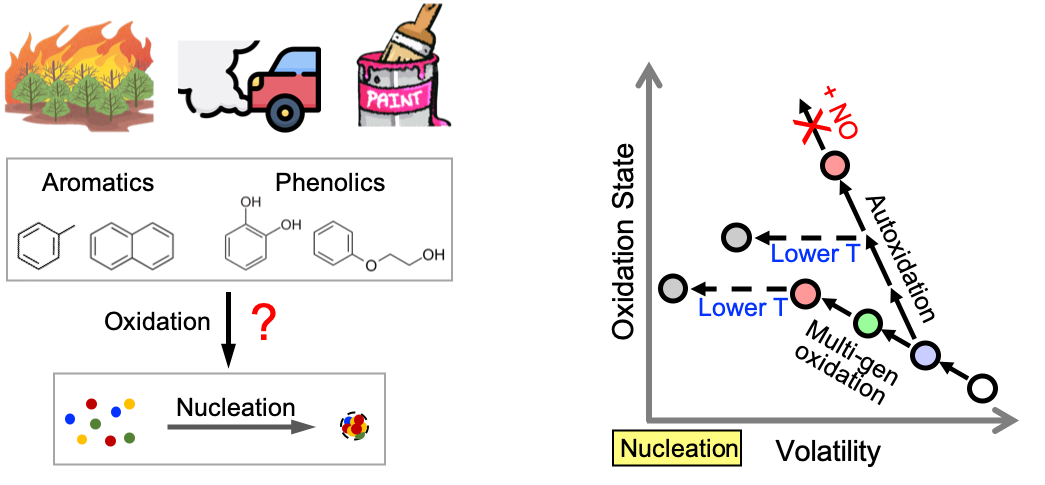
While our ambient measurements and laboratory experiments have shown that present-day nucleation in cities may be dominated by sulfuric acid, the importance of anthropogenic volatile organic compounds (AVOCs) in urban nucleation is likely to increase in the future as clean energy pledges continue to reduce SO2 emissions sharply. However, AVOCs oxidation mechanisms have challenged atmospheric kineticists for decades. Therefore, our first objective focuses on the photo-oxidation of both traditional (e.g., benzene) and emerging (e.g., phenoxyethanol) AVOCs to constrain their chemical mechanisms and product volatility distributions. We have revealed that photo-oxidation of some AVOCs can be particularly effective in producing low-volatility products that drive particle formation. Our results have provided a reference guide for volatility parameterizations to several subsequent ambient studies.
Xiao, S.; Wang, M.; Yao, L.; Kulmala, M.; Zhou, B.; Yang, X.; … Wang, L. (2015). Strong atmospheric new particle formation in winter in urban Shanghai, China. Atmos. Chem. Phys., 15, 1769–1781.[DOI]
Yao, L.; Garmash, O.; Bianchi, F.; Zheng, J.; Yan, C.; … Wang, M.; … Wang, L. (2018). Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science, 361, 278–281.[DOI]
Wang, M.; Chen, D.; Xiao, M.; Ye, Q.; Stolzenburg, D.; Hofbauer, V.; … Donahue, N. M. (2020). Photo-oxidation of aromatic hydrocarbons produces low-volatility organic compounds. Environ. Sci. Technol., 54, 7911–7921.[DOI]
Nie, W.; Yan, C.; Huang, D.; Wang, Z.; Liu, Y.; … Wang, M.; … Ding, A. (2022). Secondary organic aerosol formed by condensing anthropogenic vapours over China’s megacities. Nature Geoscience, 15, 255–261.[DOI]
Humes, M. B.; Wang, M.; Kim, S.; Machesky, J. E.; Gentner, D. R.; Robinson, A. L.; … Presto, A. A. (2022). Limited secondary organic aerosol production from acyclic oxygenated volatile chemical products. Environ. Sci. Technol., 56, 4806–4815.[DOI]
Particle growth kinetics
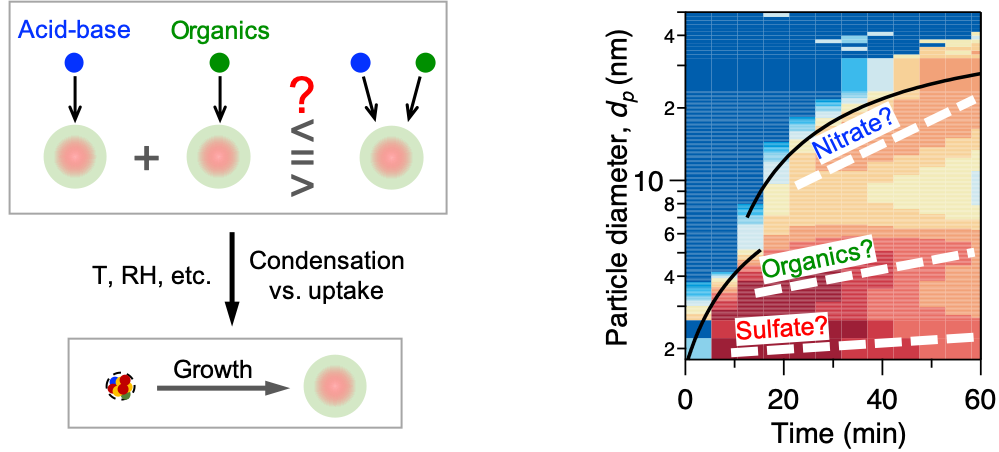
Particle formation contributes to about half of the global clouds, but its climate relevance depends largely on particle growth to sizes important for cloud physics. However, the study of initial particle growth is just beginning - we still do not know whether particle growth for different chemical systems is synergistic, parallel, or even antagonistic. Therefore, the second aim seeks to establish a correlation between vapor composition and particle growth, developing parameterizations compatible with large scale models. Our findings, featured in Nature, show that nitric acid and ammonia vapors together can drive particle growth 100 times faster than previously expected. The rapid growth rates can shepherd new particles to climate-relevant sizes where their survival against coagulation scavenging is greatly enhanced. This represents a major scientific advance, explaining puzzling observations of urban particle formation and informing control measures for haze alleviation.
Wang, M.; Kong, W.; Marten, R.; He, X.-C.; Chen, D.; Pfeifer, J.; … Donahue, N. M. (2020). Rapid growth of new atmospheric particles by nitric acid and ammonia condensation. Nature, 581, 184–189.[DOI]
Marten, R.; Xiao, M.; Rörup, B; Wang, M.; Kong, W.; He, X.-C.; … El Haddad, I. (2022). Survival of newly formed particles in haze conditions. Environ. Sci.: Atmos., 2, 491–499.[DOI]
Stolzenburg, D.; Wang, M.; Schervish, M.; Donahue, N. M. (2022). Tutorial: Dynamic organic growth modeling with a volatility basis set. J. Aero. Sci., 106063.[DOI]
Particle dynamics model and machine learning
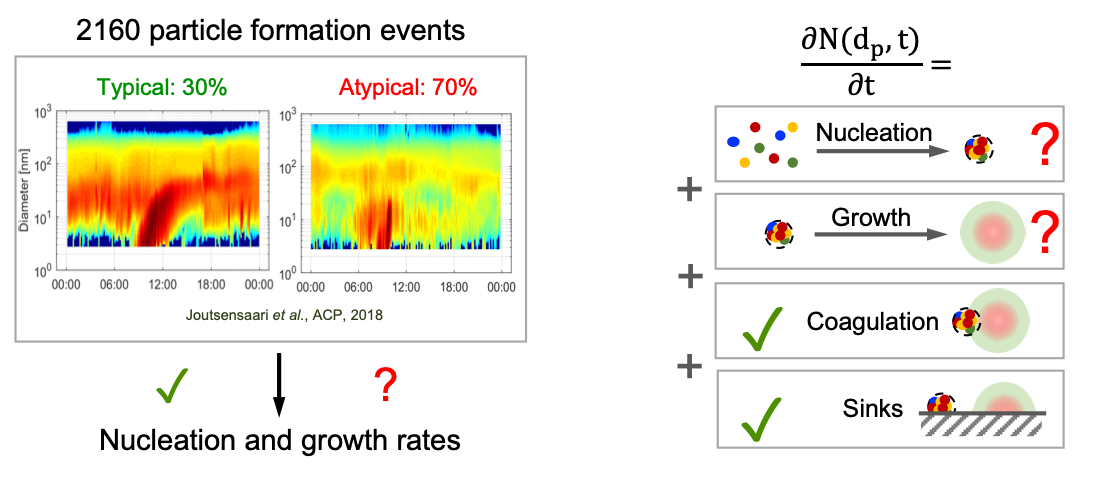
Particle size distributions are at the heart of aerosol-cloud-climate interactions, directly controlling particle scattering and cloud seeding properties. However, we lack approaches to constrain the particle dynamics of this and many other convective overshooting events, and thus their climate impacts in the upper atmosphere. Therefore, the third aim seeks to develop a particle dynamics model that incorporates particle formation parameterizations and transient environmental conditions. Some of our first results, recently published in Nature, show that nitric acid, sulfuric acid, and ammonia synergistically nucleate particles, increasing the particle population by a factor of five during the Asian monsoon. This new mechanism explains the enhanced particle and cloud layer observed over the mid-latitudes of the Northern Hemisphere, which can be studied as an observational analog of climate intervention.
Wang, M.; Xiao, M.; Bertozzi, B; Marie, G; Rörup, B; Schulze, B; … Donahue, N. M. (2022). Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation. Nature, 605, 483–489.[DOI]
